EXONDYS 51 (eteplirsen)
EXONDYS 51 is indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the DMD gene that is amenable to exon 51 skipping. This indication is approved under accelerated approval based on an increase in dystrophin in skeletal muscle observed in some patients treated with EXONDYS 51. Continued approval for this indication may be contingent upon verification of a clinical benefit in confirmatory trials.1
Designed to Skip Exon 51
EXONDYS 51 is designed to skip exon 51, correcting the out-of-frame mutation that causes a lack of functional dystrophin and intended to allow for the production of an internally truncated dystrophin protein in patients living with DMD.1-3
The effect of EXONDYS 51 on dystrophin production was evaluated in three studies in patients with a confirmed mutation of the DMD gene that is amenable to exon 51 skipping.1
EXONDYS 51 modified gene expression in all patients evaluated, as measured by RT-PCR
100% (N=36)
of patients biopsied demonstrated exon skipping with EXONDYS 511
RT-PCR=reverse transcription polymerase chain reaction.
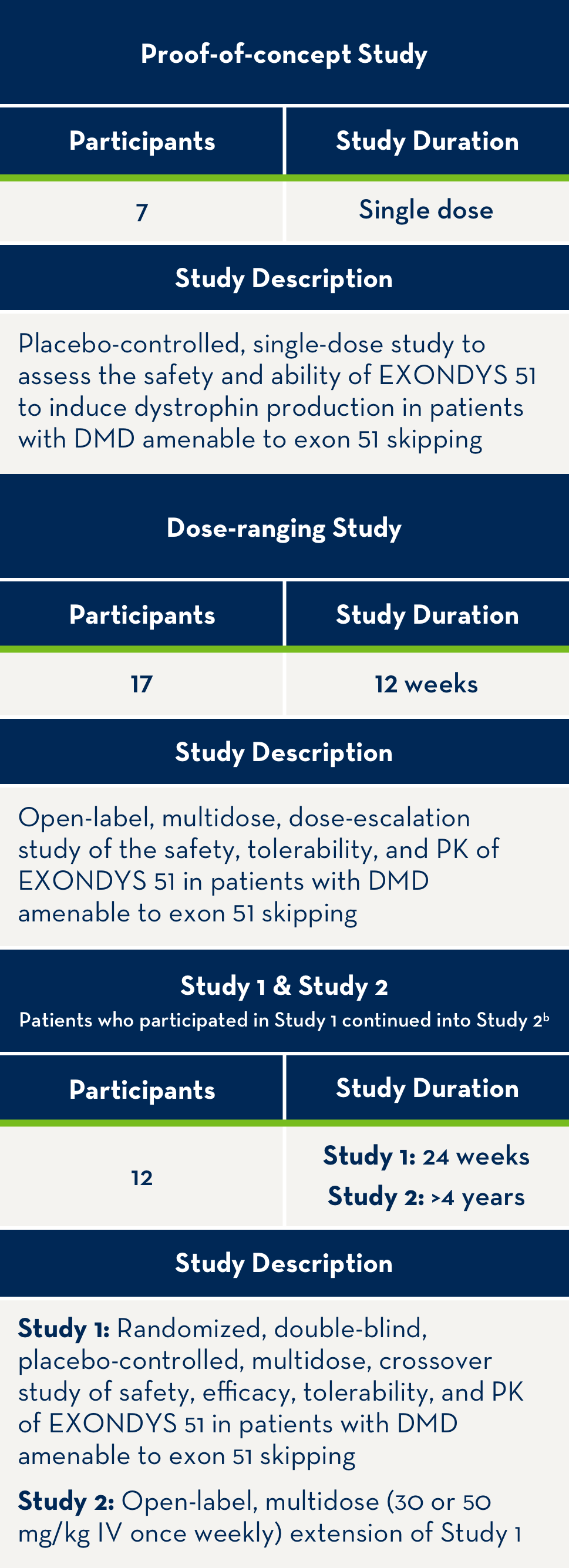
Overview of patients treated with EXONDYS 51 in clinical trials4,a
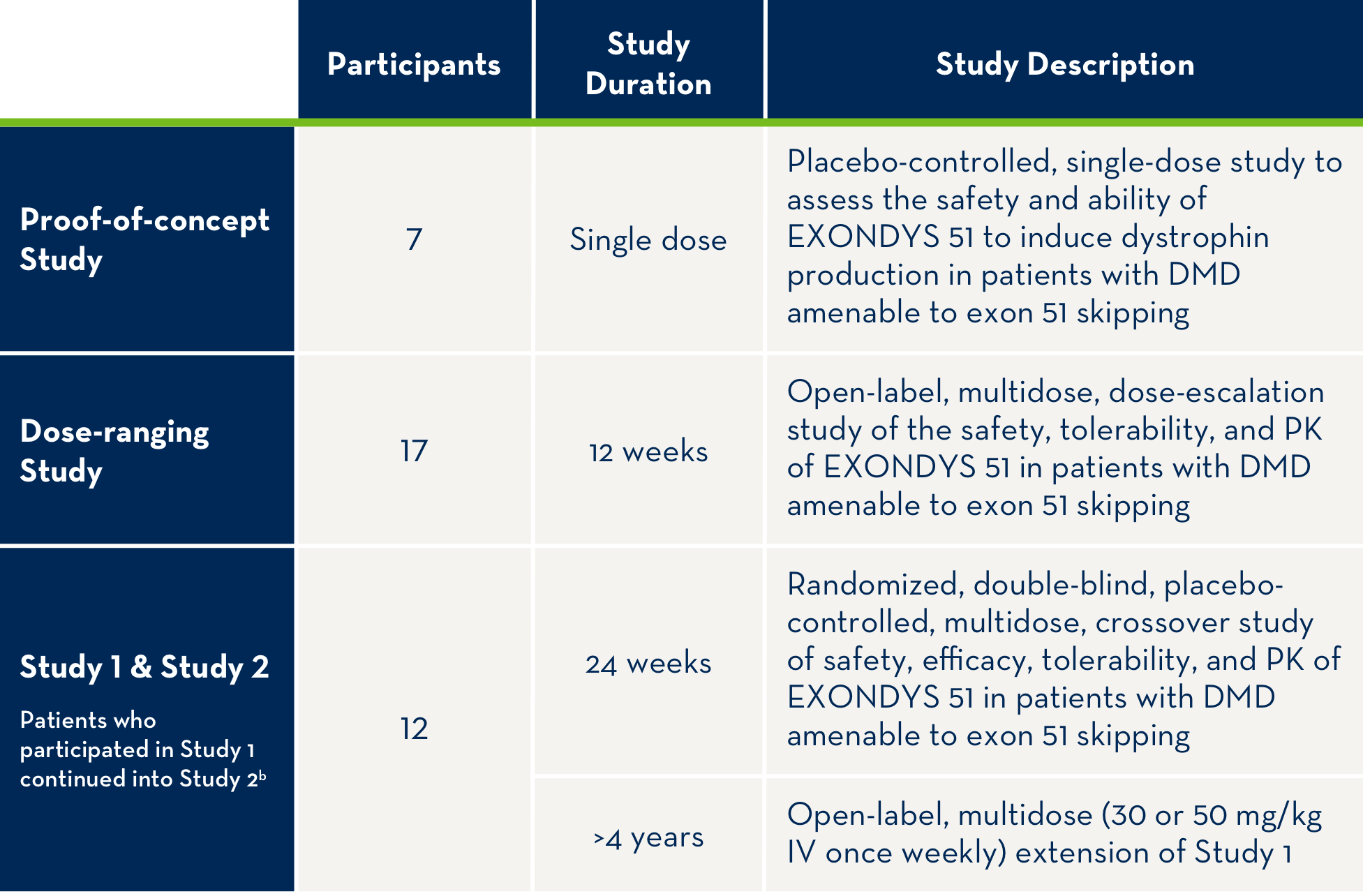
aAll patients in clinical trials have a confirmed mutation of the DMD gene that is amenable to exon 51 skipping.
bAll 12 patients who participated in Study 1 continued treatment with EXONDYS 51 weekly for an additional 4 years in Study 2.
PK=pharmacokinetics.
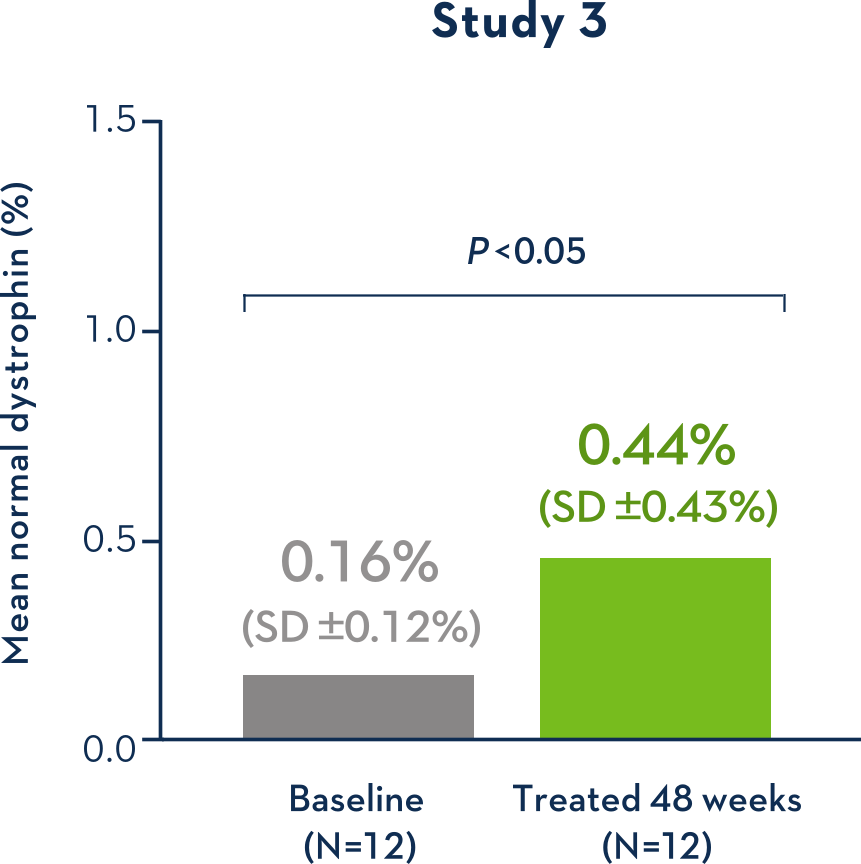
Some Patients Had an Increase in Dystrophin Over Baseline With EXONDYS 511
Patients showed an average dystrophin increase of 2.8x the baseline level (range: -0.19 to +8.40)
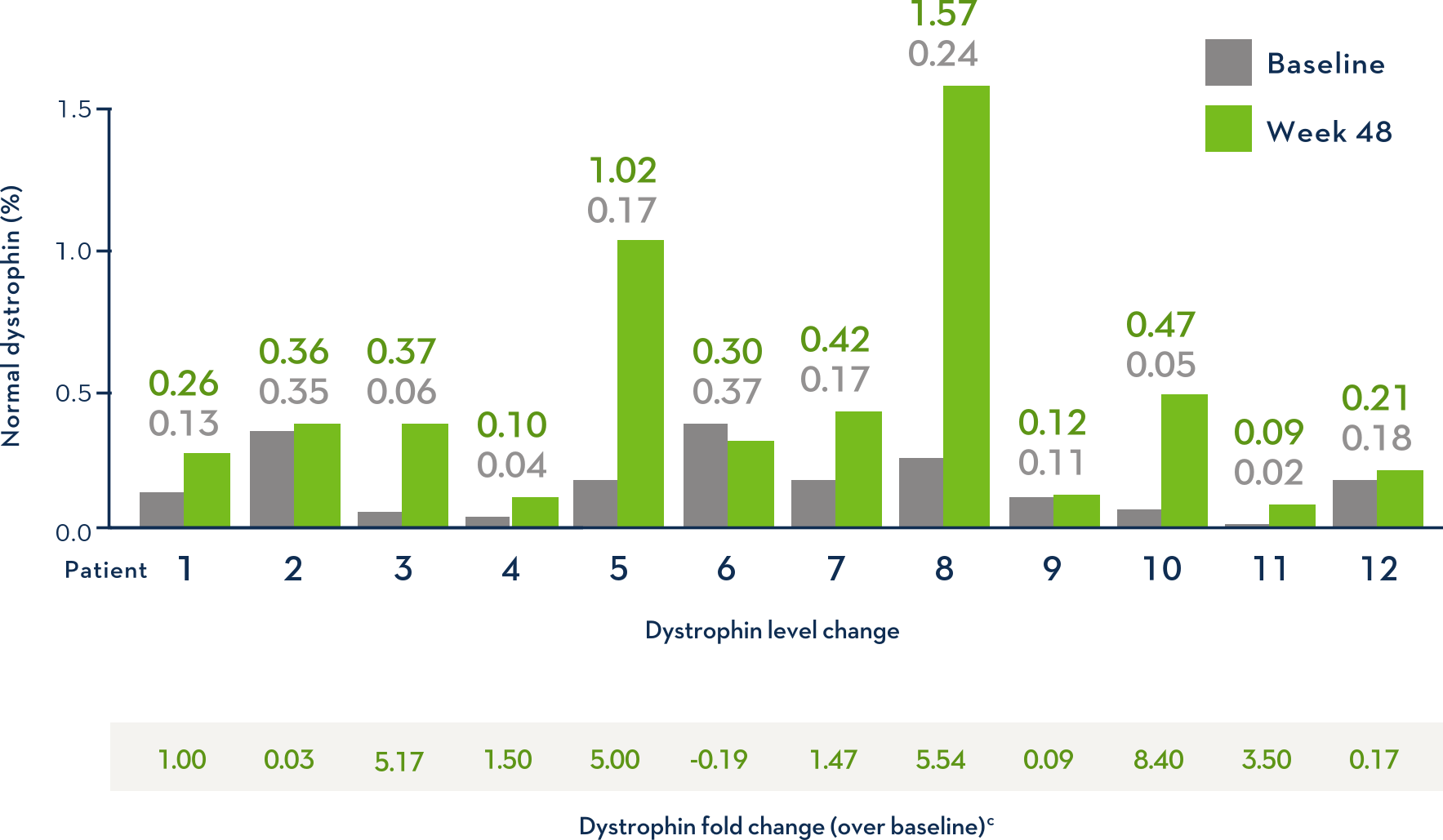
cFold change in dystrophin=change from baseline, percent normal dystrophin/baseline, percent normal dystrophin.
- Dystrophin levels measured via western blot can be affected by differences in sample processing, analytical technique, reference materials, and quantification methodologies1
- Comparing dystrophin results from different assay protocols will require a standardized reference material and additional bridging studies1
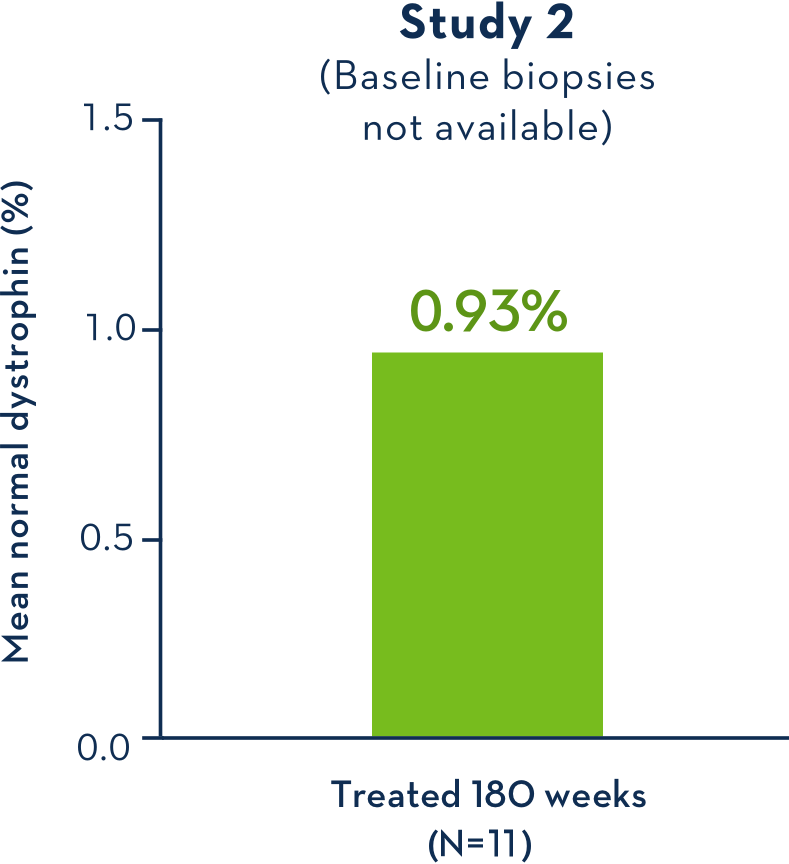
Dystrophin Production With EXONDYS 511
Average Dystrophin Protein Level in Muscle Tissue
- Dystrophin levels measured via western blot can be affected by differences in sample processing, analytical technique, reference materials, and quantification methodologies1
- Comparing dystrophin results from different assay protocols will require a standardized reference material and additional bridging studies1
- Study 2 failed to provide evidence of a clinical benefit of EXONDYS 51 compared to the external control group1
Hypersensitivity Reactions
Hypersensitivity reactions, including bronchospasm, chest pain, cough, tachycardia and urticaria, have occurred in patients who were treated with EXONDYS 51. If a hypersensitivity reaction occurs, institute appropriate medical treatment and consider slowing the infusion or interrupting the EXONDYS 51 therapy.1
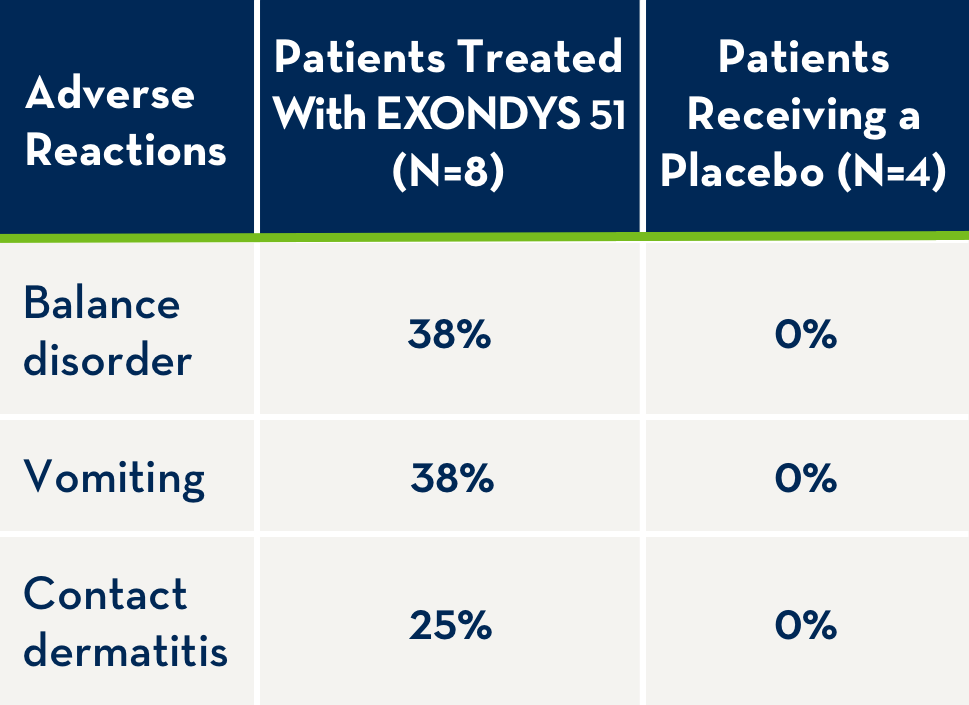
Adverse Reactions
EXONDYS 51 was studied in a double-blind, placebo-controlled study for 24 weeks (Study 1), followed by an open-label extension (Study 2). In Study 1, 12 patients were randomized to receive weekly intravenous infusions of EXONDYS 51 (N=8) or placebo (N=4) for 24 weeks. All 12 patients continued in Study 2 and received open-label EXONDYS 51 weekly for up to 208 weeks.1
The following adverse reactions were observed in patients with DMD treated for 24 weeks with 30 or 50 mg/kg/week EXONDYS 51 with incidence at least 25% more than placebo.1
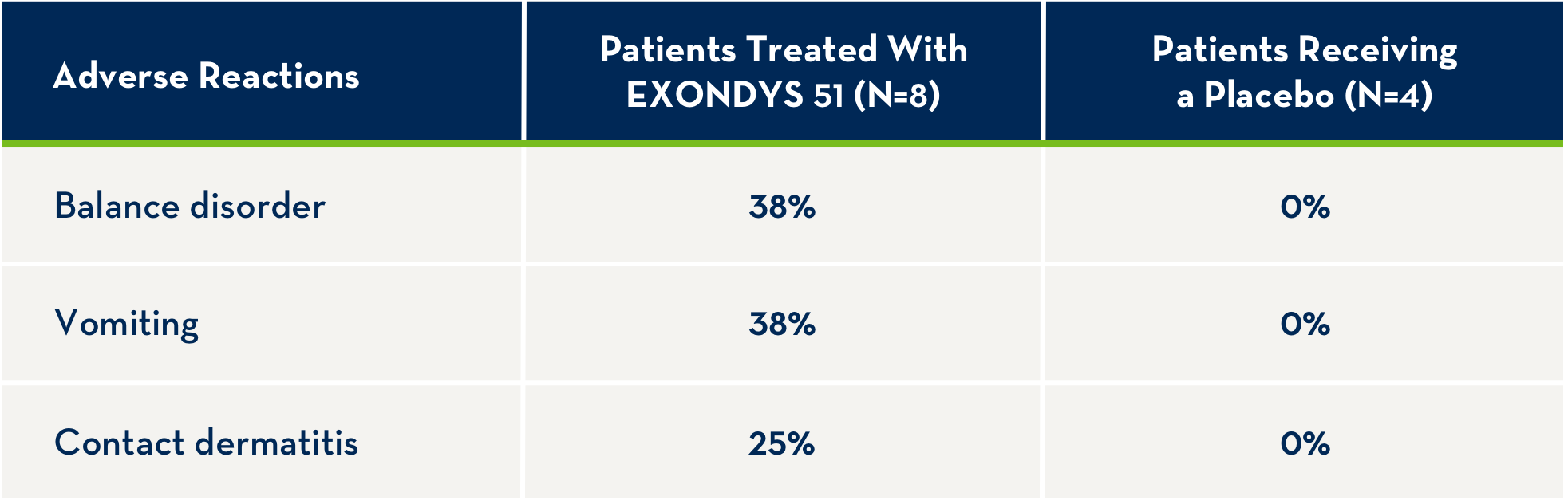
- Because of the small number of patients, these represent crude frequencies that may not reflect the frequencies observed in practice1
- The 50 mg/kg weekly dosing regimen of EXONDYS 51 is not recommended1
In open-label observational studies, 163 patients received at least one intravenous dose of EXONDYS 51, with doses ranging between 0.5 mg/kg (0.017 times the recommended dosage) and 50 mg/kg (1.7 times the recommended dosage). All patients were male and had genetically confirmed Duchenne muscular dystrophy. Age at study entry was 6 months to 19 years. Most (85%) patients were Caucasian.1
The most common adverse reactions seen in greater than 10% of the study population were headache, cough, rash, and vomiting. Postmarketing adverse reactions that occurred during infusion include bronchospasm, cyanosis of the lips, and malaise. The following adverse reactions have also been reported in patients receiving EXONDYS 51: pyrexia, flushing, protein urine present, and dehydration.1
Before administration, please see the full Prescribing Information for EXONDYS 51 (eteplirsen).
DMD Case Study
Meet James, a 13-year-old patient with DMD
This is a hypothetical case study and is solely intended for educational purposes and should not be relied on for medical advice.
Patient Profile5-16
Patient History and Presentation
| James was diagnosed with DMD at 5 years old |
| Genetic mutation analysis showed the presence of an out-of-frame deletion that is amenable to exon 51 skipping |
| James started treatment with daily prednisone at age 6 and switched to deflazacort when he was 10 years old |
| James was started on preventative cardiomyopathy therapy with an ACE inhibitor at 10 years old and a beta-blocker at 12 years old |
| James presented to his neurologist for his bi-annual visit at 13 years old, seeking assistive devices for getting around school |
| Current health stage: Late ambulatory |
Current Demographics
| Age | Height | Weight |
|---|---|---|
| 13 | 59 inches | 60 kg |
Current Medication
| Medication | Dosage |
|---|---|
| Lisinopril 5 mg | 5 mg daily |
| Carvedilol 6.25 mg | 1 tablet (6.25 mg) twice daily |
| Deflazacort 18 mg | 18 mg daily |
| Deflazacort 36 mg | 36 mg daily (with 18 mg tablet) |
Functional Tests at Last Visit (age 13)
| Exam | Result |
|---|---|
| 6-minute walk test (6MWT) | 320 m |
| North Star Ambulatory Assessment (NSAA) | 9 |
| Time to rise from floor (TRF) | Unable to complete due to loss of ability to rise |
| Performance of Upper Limb (PUL) score | 36 |
| Forced vital capacity (FVC), % predicted | 82 |
| Left ventricular ejection fraction (LVEF), % | 56 |
ACE=angiotensin-converting enzyme.
At the last visit, James was started on exon-skipping therapy with EXONDYS 51 30 mg/kg weekly1
Answer these questions to find out how colleagues have responded to this case
How important is the potential ability to produce truncated dystrophin to your decision to start a patient on an exon skipping therapy, if at all?
In your opinion, which factors of EXONDYS 51 therapy are most appealing to patients, if any?
Would you continue this patient on EXONDYS 51 once they become nonambulatory?
How representative is James of the patients you treat, if at all?
Responses from a quantitative DMD market research survey involving 25 neurologists experienced in treating DMD patients.
IMPORTANT SAFETY INFORMATION
EXONDYS 51 (eteplirsen) is indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the DMD gene that is amenable to exon 51 skipping. This indication is approved under accelerated approval based on an increase in dystrophin in skeletal muscle observed in some patients treated with EXONDYS 51. Continued approval for this indication may be contingent upon verification of a clinical benefit in confirmatory trials.
IMPORTANT SAFETY INFORMATION
Hypersensitivity:
Hypersensitivity reactions, including bronchospasm, chest pain, cough, tachycardia and urticaria, have occurred in patients who were treated with EXONDYS 51. If a hypersensitivity reaction occurs, institute appropriate medical treatment and consider slowing the infusion or interrupting the EXONDYS 51 therapy.
Adverse Reactions:
Adverse reactions in DMD patients (N=8) treated with EXONDYS 51 30 or 50 mg/kg/week by intravenous (IV) infusion with an incidence of at least 25% more than placebo (N=4) (Study 1, 24 weeks) were (EXONDYS 51, placebo): balance disorder (38%, 0%), vomiting (38%, 0%) and contact dermatitis (25%, 0%). The most common adverse reactions were balance disorder and vomiting. Because of the small numbers of patients, these represent crude frequencies that may not reflect the frequencies observed in practice. The 50 mg/kg once weekly dosing regimen of EXONDYS 51 is not recommended.
The most common adverse reactions from observational clinical studies (N=163) seen in greater than 10% of patients were headache, cough, rash, and vomiting.
Before administration, please see the full Prescribing Information for EXONDYS 51 (eteplirsen).
INDICATION
EXONDYS 51 (eteplirsen) is indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the DMD gene that is amenable to exon 51 skipping. This indication is approved under accelerated approval based on an increase in dystrophin in skeletal muscle observed in some patients treated with EXONDYS 51. Continued approval for this indication may be contingent upon verification of a clinical benefit in confirmatory trials.
References: 1. EXONDYS 51 [package insert]. Cambridge, MA: Sarepta Therapeutics, Inc. 2022. 2. Aartsma-Rus A, Van Deutekom JCT, Fokkema IF, et al. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve. 2006;34(2):135-144. 3. Wood MJA, Gait MJ, Yin H. RNA-targeted splice-correction therapy for neuromuscular disease. Brain. 2010;133(pt 4):957-972. 4. Data on file. Sarepta Therapeutics, Inc. 5. Ciafaloni E, Fox DJ, Pandya S, et al. Delayed diagnosis in Duchenne muscular dystrophy: data from the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet). J Pediatr. 2009;155(3):380-385. 6. Alfano LN, Charleston JS, Connolly AM, et al. Long-term treatment with eteplirsen in nonambulatory patients with Duchenne muscular dystrophy. Medicine (Baltimore). 2019;98(26):e15858. 7. Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018;17(3):251-267. 8. Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018;17(4):347-361. 9. Mercuri E, Signorovitch JE, Swallow E, et al; DMD Italian Group; Trajectory Analysis Project (cTAP). Categorizing natural history trajectories of ambulatory function measured by the 6-minute walk distance in patients with Duchenne muscular dystrophy. Neuromuscul Disord. 2016;26(9):576-583. Erratum in: Neuromuscul Disord. 2017;27(5):e1. 10. McDonald CM, Henricson EK, Abresch RT, et al. The 6-minute walk test and other endpoints in Duchenne muscular dystrophy: longitudinal natural history observations over 48 weeks from a multicenter study. Muscle Nerve. 2013;48(3):343-356. 11. Muntoni F, Domingos J, Manzur AY, et al; UK NorthStar Network. Categorising trajectories and individual item changes of the North Star Ambulatory Assessment in patients with Duchenne muscular dystrophy. PLoS One. 2019;14(9):e0221097. 12. Ricotti V, Ridout DA, Pane M, et al; UK NorthStar Clinical Network. The NorthStar Ambulatory Assessment in Duchenne muscular dystrophy: considerations for the design of clinical trials. J Neurol Neurosurg Psychiatry. 2016;87(2):149-155. 13. McDonald CM, Henricson EK, Abresch RT, et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: a prospective cohort study. Lancet. 2018;391(10119):451-461. 14. Pane M, Coratti G, Brogna C, et al. Longitudinal analysis of PUL 2.0 domains in ambulant and non-ambulant Duchenne muscular dystrophy patients: how do they change in relation to functional ability? J Neuromuscul Dis. 2023;10(4):567-574. 15. McDonald CM, Gordish-Dressman H, Henricson EK, et al; CINRG investigators for PubMed. Longitudinal pulmonary function testing outcome measures in Duchenne muscular dystrophy: long-term natural history with and without glucocorticoids. Neuromuscul Disord. 2018;28(11):897-909. 16. Power A, Poonja S, Disler D, et al. Echocardiographic image quality deteriorates with age in children and young adults with Duchenne muscular dystrophy. Front Cardiovasc Med. 2017;4:82.
This information is intended for U.S. healthcare professionals only.