VYONDYS 53 (golodirsen)
VYONDYS 53 is indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the DMD gene that is amenable to exon 53 skipping. This indication is approved under accelerated approval based on an increase in dystrophin production in skeletal muscle observed in patients treated with VYONDYS 53. Continued approval for this indication may be contingent upon verification of a clinical benefit in confirmatory trials.1
Designed to Skip Exon 53
VYONDYS 53 is designed to skip exon 53, correcting the out-of-frame mutation that causes a lack of functional dystrophin and intended to allow for the production of an internally truncated dystrophin protein in patients living with DMD.1-3
The effect of VYONDYS 53 on dystrophin production was evaluated in two studies in patients with a confirmed mutation of the DMD gene that is amenable to exon 53 skipping.1
VYONDYS 53 modified gene expression in all patients evaluated, as measured by RT-PCR
100% (N=25)
of patients biopsied demonstrated exon skipping with VYONDYS 531
RT-PCR=reverse transcription polymerase chain reaction.
Overview of patients treated with VYONDYS 53 in clinical trials1,a

aAll patients in clinical trials have a confirmed mutation of the DMD gene that is amenable to exon 53 skipping.
bMoA confirmed by RT-PCR.
MoA=mechanism of action.
mRNA=messenger ribonucleic acid.
100% of Patients Treated With VYONDYS 53 Increased Dystrophin Expression Over Baseline1
Patients treated with VYONDYS 53 showed an average dystrophin increase of 15.96 fold4
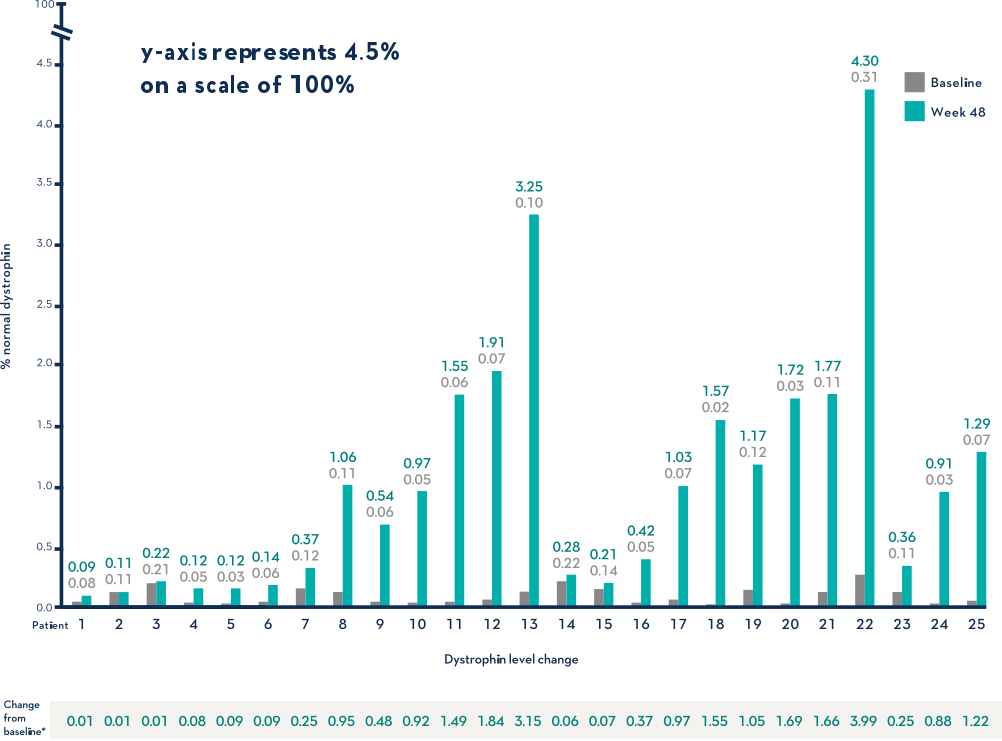
Study 1 (Part 2): Dystrophin Expression From Baseline to Week 48 (n=25)1
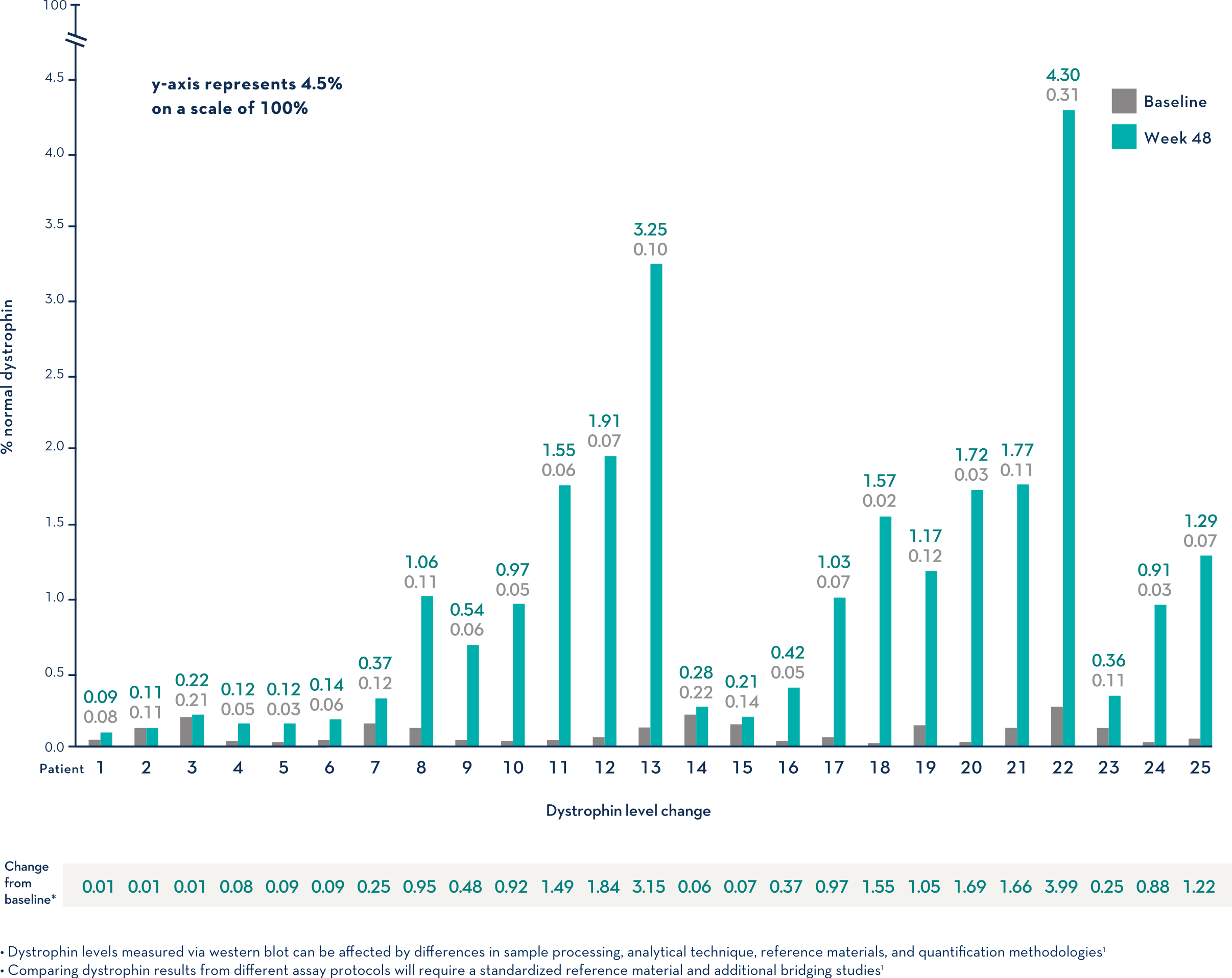
- Dystrophin levels measured via western blot can be affected by differences in sample processing, analytical technique, reference materials, and quantification methodologies1
- Comparing dystrophin results from different assay protocols will require a standardized reference material and additional bridging studies1
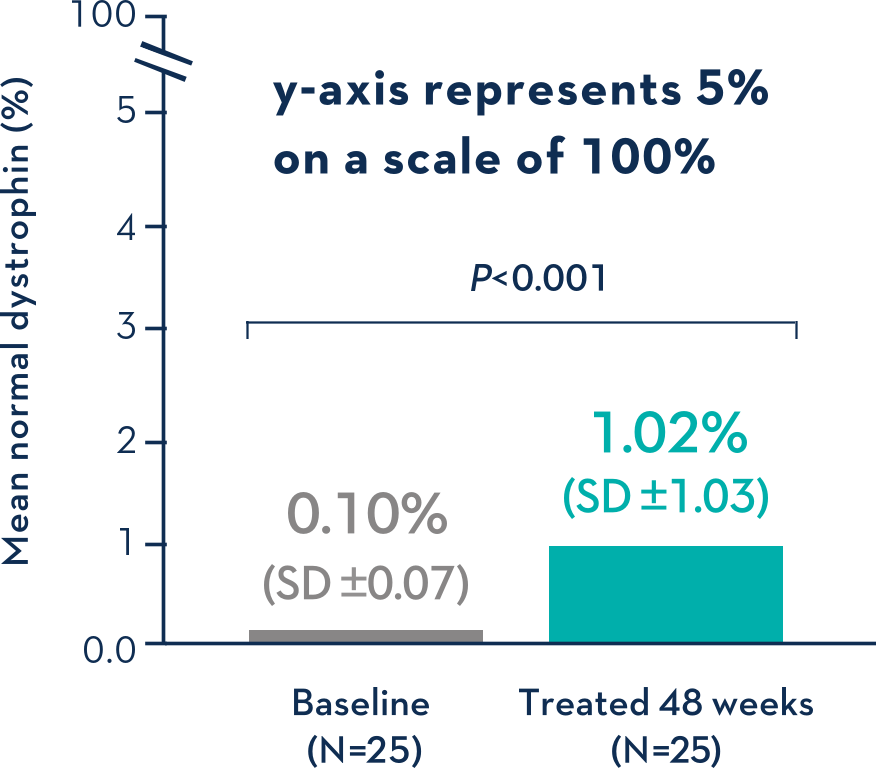
Patients Treated With VYONDYS 53 Had a Mean Change in Dystrophin Levels of 0.92% at 48 Weeks1
Average Dystrophin Protein Level in Muscle Tissue After 48 Weeks on VYONDYS 531
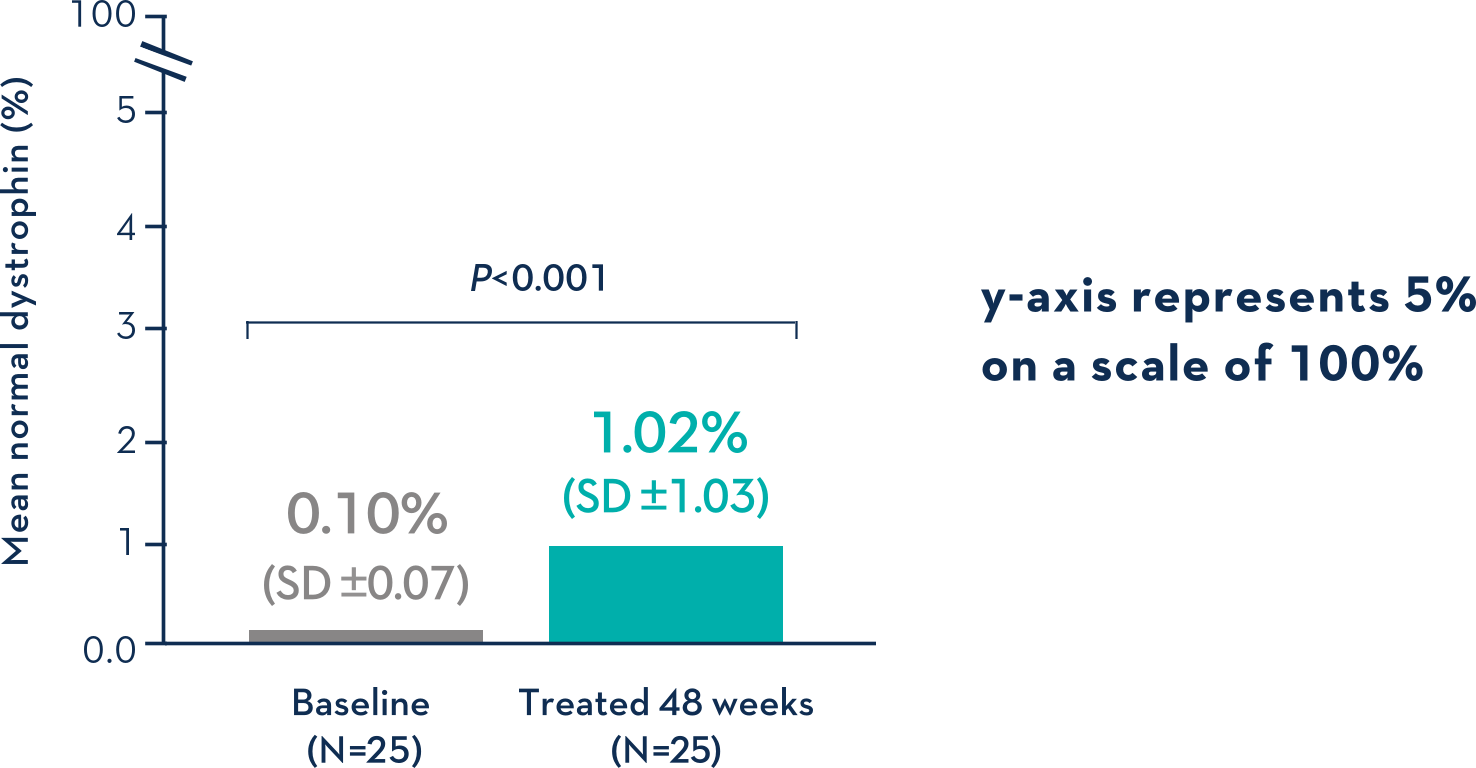
- Dystrophin levels measured via western blot can be affected by differences in sample processing, analytical technique, reference materials, and quantification methodologies1
- Comparing dystrophin results from different assay protocols will require a standardized reference material and additional bridging studies1
Contraindications
VYONDYS 53 is contraindicated in patients with a serious hypersensitivity reaction to golodirsen or to any of the inactive ingredients in VYONDYS 53. Anaphylaxis has occurred in patients receiving VYONDYS 53.1
Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis, rash, pyrexia, pruritus, urticaria, dermatitis, and skin exfoliation have occurred in VYONDYS 53-treated patients, some requiring treatment. If a hypersensitivity reaction occurs, institute appropriate medical treatment and consider slowing the infusion, interrupting, or discontinuing the VYONDYS 53 therapy and monitor until the condition resolves. VYONDYS 53 is contraindicated in patients with a history of a serious hypersensitivity reaction to golodirsen or to any of the inactive ingredients in VYONDYS 53.1
Kidney Toxicity
Kidney toxicity was observed in animals who received golodirsen. Although kidney toxicity was not observed in the clinical studies with VYONDYS 53, the clinical experience with VYONDYS 53 is limited, and kidney toxicity, including potentially fatal glomerulonephritis, has been observed after administration of some antisense oligonucleotides. Kidney function should be monitored in patients taking VYONDYS 53. Because of the effect of reduced skeletal muscle mass on creatinine measurements, creatinine may not be a reliable measure of kidney function in DMD patients. Serum cystatin C, urine dipstick, and urine protein-to-creatinine ratio should be measured before starting VYONDYS 53. Consider also measuring glomerular filtration rate using an exogenous filtration marker before starting VYONDYS 53. During treatment, monitor urine dipstick every month, and serum cystatin C and urine protein-to-creatinine ratio every three months. Only urine expected to be free of excreted VYONDYS 53 should be used for monitoring of urine protein. Urine obtained on the day of VYONDYS 53 infusion prior to the infusion, or urine obtained at least 48 hours after the most recent infusion, may be used. Alternatively, use a laboratory test that does not use the reagent pyrogallol red, as this reagent has the potential to cross-react with any VYONDYS 53 that is excreted in the urine and thus lead to a false positive result for urine protein.1
If a persistent increase in serum cystatin C or proteinuria is detected, refer to a pediatric nephrologist for further evaluation.1
Adverse Reactions
In the VYONDYS 53 clinical development program, 58 patients received at least one intravenous dose of VYONDYS 53, ranging between 4 mg/kg (0.13 times the recommended dosage) and 30 mg/kg (the recommended dosage). All patients were male and had genetically confirmed DMD. Age at study entry was 6 to 13 years. Most (86%) patients were Caucasian.1
VYONDYS 53 was studied in 2 double-blind, placebo-controlled studies.1
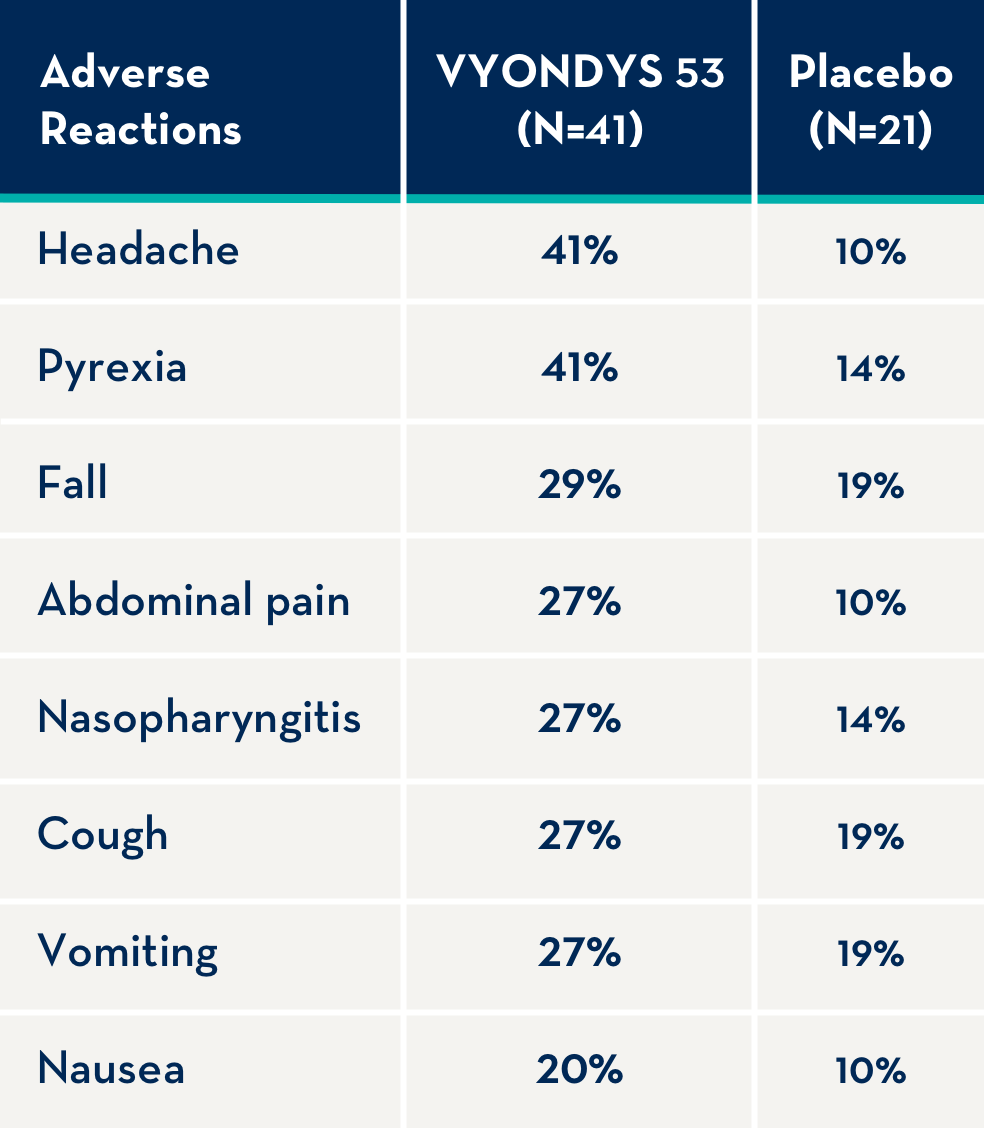
The following adverse reactions occurred in at least 20% of VYONDYS 53-treated patients and at a rate greater than placebo.1
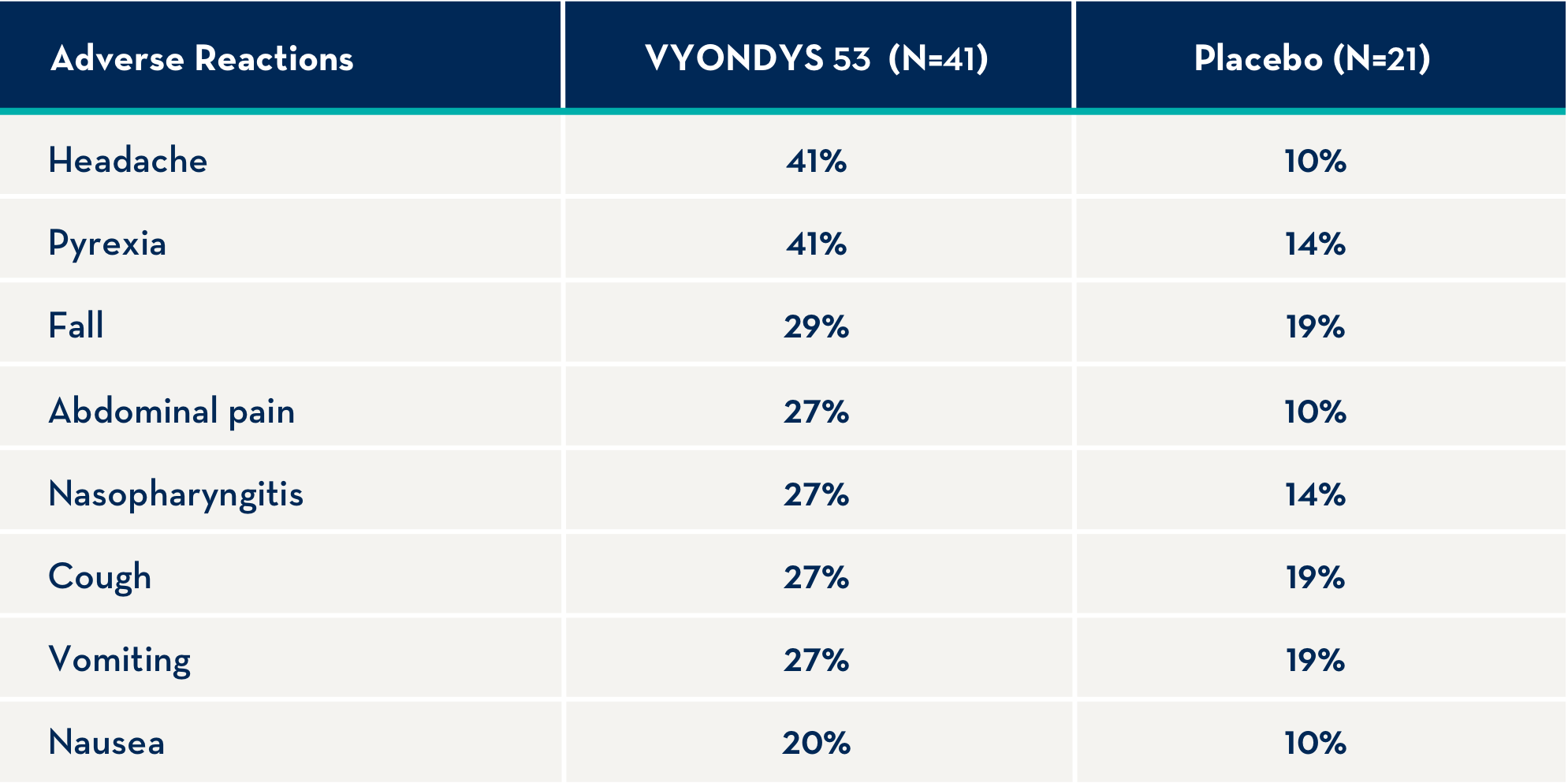
Other adverse reactions that occurred at a frequency greater than 5% of VYONDYS 53-treated patients and at a greater frequency than placebo were: administration site pain, back pain, pain, diarrhea, dizziness, ligament sprain, contusion, influenza, oropharyngeal pain, rhinitis, skin abrasion, ear infection, seasonal allergy, tachycardia, catheter site related reaction, constipation, and fracture.1
Other adverse events may occur.
To report SUSPECTED ADVERSE REACTIONS, contact Sarepta Therapeutics, Inc. at 1-888-SAREPTA (1-888-727-3782) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Before administration, please see the full US Prescribing Information for VYONDYS 53 (golodirsen).
DMD Case Study
Meet Ben, a 15-year-old patient with DMD
This is a hypothetical case study and is solely intended for educational purposes and should not be relied on for medical advice.
Patient Profile1,5-12
Patient History and Presentation
| Ben was diagnosed with DMD at 5 years old |
| Genetic mutation analysis showed the presence of an out-of-frame deletion that is amenable to exon 53 skipping |
| Ben started treatment with daily prednisone at age 6 |
| Ben was started on preventative cardiomyopathy therapy with an ACE inhibitor at 11 years old and a mineralocorticoid receptor antagonist at 14 years old |
| Ben reported increased stiffness, fatigue, and myalgia at his last Neurology appointment 6 months ago |
| Ben lost ambulation 22 months ago |
| Current health stage: Early nonambulatory |
Current Demographics
| Age | Height | Weight |
|---|---|---|
| 15 | 66 inches | 77 kg |
Current Medication
| Medication | Dosage |
|---|---|
| Lisinopril 5 mg | 5 mg daily |
| Spironolactone 25 mg | 25 mg daily |
| Prednisone 25 mg | 2 tablets (50 mg) daily |
Functional Tests at Last Visit (age 15)
| Exam | Result |
|---|---|
| Performance of Upper Limb (PUL) score | 28 |
| Forced vital capacity (FVC), % predicted | 60 |
| Left ventricular ejection fraction (LVEF), % | 53 |
ACE=angiotensin-converting enzyme.
At the last visit, Ben was started on exon-skipping therapy with VYONDYS 53 30 mg/kg once weekly1
Answer these questions to find out how colleagues have responded to this case
How important is the potential ability to produce truncated dystrophin to your decision to start a patient on an exon skipping therapy, if at all?
In your opinion, which factors of VYONDYS 53 therapy are most appealing to patients, if any?
How representative is Ben of the patients you treat, if at all?
Responses from a quantitative DMD market research survey involving 25 neurologists experienced in treating DMD patients.
IMPORTANT SAFETY INFORMATION
VYONDYS 53 is indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the DMD gene that is amenable to exon 53 skipping. This indication is approved under accelerated approval based on an increase in dystrophin production in skeletal muscle observed in patients treated with VYONDYS 53. Continued approval for this indication may be contingent upon verification of a clinical benefit in confirmatory trials.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS:
VYONDYS 53 is contraindicated in patients with a serious hypersensitivity reaction to golodirsen or to any of the inactive ingredients in VYONDYS 53. Anaphylaxis has occurred in patients receiving VYONDYS 53.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions:
Hypersensitivity reactions, including anaphylaxis, rash, pyrexia, pruritus, urticaria, dermatitis, and skin exfoliation have occurred in VYONDYS 53-treated patients, some requiring treatment. If a hypersensitivity reaction occurs, institute appropriate medical treatment and consider slowing the infusion, interrupting, or discontinuing the VYONDYS 53 therapy and monitor until the condition resolves. VYONDYS 53 is contraindicated in patients with a history of a serious hypersensitivity reaction to golodirsen or to any of the inactive ingredients in VYONDYS 53.
Kidney Toxicity:
Kidney toxicity was observed in animals who received golodirsen. Although kidney toxicity was not observed in the clinical studies with VYONDYS 53, the clinical experience with VYONDYS 53 is limited, and kidney toxicity, including potentially fatal glomerulonephritis, has been observed after administration of some antisense oligonucleotides. Kidney function should be monitored in patients taking VYONDYS 53. Because of the effect of reduced skeletal muscle mass on creatinine measurements, creatinine may not be a reliable measure of kidney function in DMD patients. Serum cystatin C, urine dipstick, and urine protein-to-creatinine ratio should be measured before starting VYONDYS 53. Consider also measuring glomerular filtration rate using an exogenous filtration marker before starting VYONDYS 53. During treatment, monitor urine dipstick every month, and serum cystatin C and urine protein-to-creatinine ratio every three months. Only urine expected to be free of excreted VYONDYS 53 should be used for monitoring of urine protein. Urine obtained on the day of VYONDYS 53 infusion prior to the infusion, or urine obtained at least 48 hours after the most recent infusion, may be used. Alternatively, use a laboratory test that does not use the reagent pyrogallol red, as this reagent has the potential to cross-react with any VYONDYS 53 that is excreted in the urine and thus lead to a false positive result for urine protein.
If a persistent increase in serum cystatin C or proteinuria is detected, refer to a pediatric nephrologist for further evaluation.
Adverse Reactions:
Adverse reactions observed in at least 20% of treated patients and greater than placebo were (VYONDYS 53, placebo): headache (41%, 10%), pyrexia (41%, 14%), fall (29%, 19%), abdominal pain (27%, 10%), nasopharyngitis (27%, 14%), cough (27%, 19%), vomiting (27%, 19%), and nausea (20%, 10%).
Other adverse reactions that occurred at a frequency greater than 5% of VYONDYS 53-treated patients and at a greater frequency than placebo were: administration site pain, back pain, pain, diarrhea, dizziness, ligament sprain, contusion, influenza, oropharyngeal pain, rhinitis, skin abrasion, ear infection, seasonal allergy, tachycardia, catheter site related reaction, constipation, and fracture.
Other adverse events may occur.
To report SUSPECTED ADVERSE REACTIONS, contact Sarepta Therapeutics, Inc. at 1-888-SAREPTA (1-888-727-3782) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Before administration, please see the full US Prescribing Information for VYONDYS 53 (golodirsen).
INDICATIONS AND USAGE
VYONDYS 53 is indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the DMD gene that is amenable to exon 53 skipping. This indication is approved under accelerated approval based on an increase in dystrophin production in skeletal muscle observed in patients treated with VYONDYS 53. Continued approval for this indication may be contingent upon verification of a clinical benefit in confirmatory trials.
References: 1. VYONDYS 53 [package insert]. Cambridge, MA: Sarepta Therapeutics, Inc. 2024. 2. Aartsma-Rus A, Van Deutekom JCT, Fokkema IF, et al. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve. 2006;34(2):135-144. 3. Wood MJA, Gait MJ, Yin H. RNA-targeted splice-correction therapy for neuromuscular disease. Brain. 2010;133(pt 4):957-972. 4. Frank DE, Mercuri E, Servais L, et al. Golodirsen induces exon skipping leading to sarcolemmal dystrophin expression in patients with genetic mutations amenable to exon 53 skipping. Poster presented at: Annual Clinical Genetics Meeting of the American College of Medical Genetics and Genomics; April 2-6, 2019; Seattle, Washington. 5. Ciafaloni E, Fox DJ, Pandya S, et al. Delayed diagnosis in Duchenne muscular dystrophy: data from the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet). J Pediatr. 2009;155(3):380-385. 6. Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018;17(3):251-267. 7. Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018;17(4):347-361. 8. Houwen-van Opstal SLS, Heutinck L, Jansen M, et al. Occurrence of symptoms in different stages of Duchenne muscular dystrophy and their impact on social participation. Muscle Nerve. 2021;64(6):701-709. 9. Raman SV, Hor KN, Mazur W, et al. Stabilization of early Duchenne cardiomyopathy with aldosterone inhibition: results of the multicenter AIDMD trial. J Am Heart Assoc. 2019;8(19):e013501. 10. Pane M, Coratti G, Brogna C, et al. Longitudinal analysis of PUL 2.0 domains in ambulant and non-ambulant Duchenne muscular dystrophy patients: how do they change in relation to functional ability? J Neuromuscul Dis. 2023;10(4):567-574. 11. McDonald CM, Gordish-Dressman H, Henricson EK, et al; CINRG investigators for PubMed. Longitudinal pulmonary function testing outcome measures in Duchenne muscular dystrophy: long-term natural history with and without glucocorticoids. Neuromuscul Disord. 2018;28(11):897-909. 12. Power A, Poonja S, Disler D, et al. Echocardiographic image quality deteriorates with age in children and young adults with Duchenne muscular dystrophy. Front Cardiovasc Med. 2017;4:82. 13. Data on file. Sarepta Therapeutics, Inc.
This information is intended for U.S. healthcare professionals only.